Biolysis Weekly Issue 2
Ondansetron, a Proven Drug, with a Hidden Value - Adial Pharmaceuticals (ADIL)
If you have ever experienced a bad bout with gastrointestinal issues, constant vomiting, and feeling nauseous at the mere thought of eating or drinking, then chances are your doctor has prescribed you the antiemetic drug Ondansetron (GR 38032F) — more commonly known by its commercial brand name Zofran.
Ondansetron is a pharmaceutical drug that was first invented in the 1980s, by GlaxoSmithKline (GSK) [NYSE: GSK — $97.93 Billion], and eventually patented in the U.S., by 1986-1988. In 1991, Ondansetron was first FDA approved strictly for use as an intravenous (IV) drug, and then injection intramuscularly (IM). Oral tablets (pill and dissolvable) came later, in 1995 and 1999 (dissolvable) respectively.
Ondansetron has undergone some of the most extensive regulatory and safety trials till date, with multiple approved patents for different uses. The first patent was given in 1986 — U.S. Patent 4753789, and then another in 1988 — U.S. Patent 4929632. In 1996, GSK’s Ondansetron was also given U.S. Patent 5578628A.
Pharmaceutical patent terms last for 20 years. Given the fact Ondansetron’s first patent was first initiated in 1986, the patent for anti-emetic uses expired in 2006.
Ondansetron is a 5HT-3 receptor antagonist, which works directly on the serotonin receptors of the brain. 5HT-3 receptors mediate excitation in the central (CNS) and peripheral nervous systems (PNS). They are primarily located at the ends of the vagus nerve, and within the medulla, in an area known as the “chemoreceptor trigger zone” — due to the high traffic of neurotransmitters in the region. 90% of the body’s serotonin is actually produced in the intestines and other regions of the GI tract (particularly where enterochromaffin cells reside). So, it would make sense that there are many vagal sensory neurons in the gut. In other words, anything your gut comes in contact with (i.e., alcohol), has a direct relation to 5-HT3 receptor excitation in the brain, via vagal sensory neurons. Hence, this is why one feels “buzzed” or happy when drinking alcohol.
Since Ondansetron’s FDA approval, three more 5HT-3 receptor antagonists have also been approved. Listed, they are as follows: Granisetron, Dolasetron, and Palonosetron. All approved 5HT-3 receptor antagonists have had very minimal side-effects and have been approved by various global regulatory bodies for anti-emetic purposes, in children as young as 4, pregnant women dealing with morning sickness, as well as chemotherapy and radiologic patients. In fact, Ondansetron can even be given to pets, such as dogs and cats. So, safety of the drug at low-medium dosages does not seem to be an issue — only at high doses. It can safely be taken from 4mg orally, all the way to 16mg IV.
By 2006, Ondansetron had become one of the world’s most popular prescribed pharmaceutical drugs, and expensive at that — ranging from $800 Million to $1.4 Billion in annual sales.
Here is where things start to get interesting. As mentioned before, GSK’s Ondansetron 20 year patent period ended in 2006, which allowed for numerous biopharmaceutical companies to look towards repurposing or selling the drug in its generic form.
When a patent term to be the sole labeler of a pharmaceutical drug ends, the onus of price discovery of said pharmaceuticals falls on the first-movers who receive FDA approval for generic drug labeling and distribution. Often, this price gets cut in half or more. However, this is not always the case, as seen with the story of Martin Shkreli and his company Turin Pharmaceuticals, raising the price of Daraprim by 5,500%. Or, with Rodelis Therapeutics hiking the price of Cycloserine from $500 to $10,800.
But, I digress…
Thankfully, the price of Ondansetron was actually reduced after the patent expired. By 2007, two more companies had hopped on the Ondansetron bandwagon, to market the FDA approved generic form of the drug at a price discount — Teva Pharmaceuticals (NYSE: TEVA — $9.8 Billion) and SICOR Pharmaceuticals.
In 2012, Ondansetron offered in 32 mg IV doses was discontinued, due to “serious cardiac risks”. This is when further safety trials were done and the maximum IV dose was lowered to 16 mg, where it stands today with minimal risks.
Additionally, in 2012 GSK was found guilty, on counts of fraud and failure to report safety data, by marketing and distributing Zofran (Odansetron) for uses unapproved by the FDA at the time (i.e., morning sickness). GlaxoSmithKline paid the Department of Justice (DOJ) a $3 Billion fine. Moreover, there was also a criminal plea agreement for GSK-U.S.’s president and board of directors. Read here for the full statements, from the DOJ. Here is an excerpt:
“It further resolves allegations that GSK promoted certain forms of Zofran, approved only for post-operative nausea, for the treatment of morning sickness in pregnant women. It also includes allegations that GSK paid kickbacks to health care professionals to induce them to promote and prescribe these drugs.”
“The United States alleges that this conduct caused false claims to be submitted to federal health care programs.”
From the period of 2012-2015, GSK dealt with numerous lawsuits stemming from this event, mainly from mothers whose newborns had heart defects and other such issues, which they attributed to taking Zofran in their first trimester. Yet, not a single study has yet conclusively attributed Zofran to newborn defects. According to the CDC:
“About 7 in 10 mothers reported morning sickness during the first three months of pregnancy. Use of ondansetron by pregnant women increased from about 1 in 100 before 2000 to about 1 in 10 by 2011. For most of the birth defects studied, researchers found that taking ondansetron during early pregnancy did not appear to increase the chance of having a baby with a birth defect”.
At this stage, GSK was overrun with the avalanche of lawsuits, stemming from their 2012 settlement with the DOJ. 60 litigation suits/cases had been pending against them by mid 2015. This is likely a major reason why they decided to sell the rights of Zofran to Novartis (NYSE: NVS — $225.6 Billion) in 2015.
Since then, all of Zofran’s “Products Liabilities Litigations” (MDL 2657) have remained pending/ongoing in the judicial jurisdiction of Judge Dennis Saylor, IV in the US District Court of Massachusetts. Novartis has also been added as a defendant.
By 2017, Teva Pharmaceuticals, which had previously been FDA approved for generic marketing/distribution in 2007, strategically bought out its competitor SICOR Pharmaceuticals, which was the world’s leading generics manufacturer at the time, for $3.4 Billion.
Enter…Adial Pharmaceuticals (ADIL)
Now that I have exasperatingly discussed much about the history, pharmacodynamics and regulatory affairs of Ondansetron, let me bring your attention to what I believe to be a deeply undervalued company in the biopharmaceutical industry — especially among the “pure-play” sub $50 million market cap space.
Authors note: I truly believe the story of Ondansetron is far from over, and that there is another use of the drug which may far transcend the ROI potential that Ondansetron has ever held till date.
If you look for any studies on Ondansetron, you will begin to notice many peer-reviewed publications referring to Ondansetron, not simply as an antiemetic, but rather under the categorization domain of “psychiatric drug development”. One such study, published in the Journal of Psychiatric Practice, can be found here. This is primarily due to the fact that Ondansetron works upon the aforementioned 5HT-3 receptors, which may allow for wider clinical use in the treatment of other conditions (i.e., addictions).
In a study done in 2000, a licensed physician and board-certified psychiatrist (in Europe and the U.S.) by the name of Dr. Bankole Johnson, Dsc, MD, MPhil, FRCPsych, published a double-blind, randomized controlled trial (with placebo) in the Journal of the American Medical Association (JAMA), on early-onset alcoholism. The study’s data concluded the following:
“Ondansetron, 4 µg/kg twice per day, was superior to placebo in increasing percentage of days abstinent (70.10 vs 50.20; P = .02) and total days abstinent per study week (6.74 vs 5.92; P = .03).”
“Our results suggest that ondansetron (particularly the 4 µg/kg twice per day dosage) is an effective treatment for patients with early-onset alcoholism, presumably by ameliorating an underlying serotonergic abnormality.”
The study’s results actually make a tremendous amount of sense, since molecular studies in the past have shown “alcohol intake to potentiate selective 5-HT3 receptor-mediated ion currents, an effect blocked by selective 5-HT3 receptor antagonists”. So, it would seem that potential interventions for many forms alcoholism and similar addictions may lie within 5-HT3 receptor antagonists (i.e., Ondansetron).
Dr. Johnson had the vision of initiating a study on Ondansetron as a clinical treatment for heavy drinking in adults (AUD). He actually initiated the study in 2009, as per the National Institute of Health (NIH) clinical trials registry. At the time, Dr. Bankole Johnson was a Professor and Chairman at the University of Virginia, in the Department of Psychiatry and Neurobehavioral Sciences.
Preceding this inspirational moment, Dr. Johnson had been well known in the scientific and medical community for his research on pharmaceutical based alcohol cessation methods. In 2003, he was credited for the notable discovery of a gamma-aminobutyric acid (GABA) facilitator known as topiramate (sold under the brand name Topamax). His finding led to a pharmaceutical intervention for the treatment of epilepsy and migraines. He also studied the effect of topiramate for alcohol-cessation and dependence purposes. So, it is safe to assume Dr. Johnson may be well-versed, and even a pioneer, in the area of addiction.
Ancillary to his scientific feats, he has received global attention in his role within the HBO documentary, “Addiction”. The documentary won the Governors Award and Dr. Johnson, himself, earned an Emmy Award from the Academy of Television Arts and Sciences.
As he so often says, in many of his public interviews (such as this PBS interview here), Dr. Johnson realized that the space of Alcohol Use Disorder (AUD) did not have any viable pharmaceutical treatments, and that he would aim to fill that void by founding a full-fledged clinical-stage biopharmaceutical company out of Charlottesville, VA — Adial Pharmaceuticals (NASDAQ: ADIL — $42.01 Million). Adial Pharmaceuticals (ADIL) had its initial public offering in September of 2018.
A notable thing Dr. Johnson did was that he brought forth his patented formulation of Ondansetron for AUD purposes —AD04 (0.33mg ultra low-dose of Ondansetron) — through Phase 2b of clinical trials, prior to doing an IPO to raise money, as Phase 3 trials can be cost intensive.
Currently, ADIL is run by CEO William ‘Bill’ Stilley, a U.S. Marine, who was formerly VP of Business Development at Clinical Data, Inc. There, he was personally responsible for licensing, mergers and acquisitions, as well as the management of Phase 3 Clinical Trials for Viibryd (vilazadone HCL) — a pharmaceutical treatment intervention for major depressive disorder (MDD).
Before Clinical Data, Inc., Stilley was also the COO/CFO of Adenosine Therapeutics and had established many relations through closed acquisition deals, with Johnson & Johnson, Novartis, and many more notable companies. As one could surmise, there is a tremendous amount of experience at the helm of this company, in bringing pharmaceuticals from clinical trials to production, and even M&As.
Since Adial Pharmaceutical’s Inception, many milestone have been hit over the last three year span. Here is the timeline of notable achievements and announcements, which add to the buy-side thesis:
(July 27, 2018) Adial Pharmaceuticals (ADIL) prices NASDAQ IPO at $7.32 Million — 1.46 million public shares offered at $5 per share. Warrants are also offered (ADILW). IPO date — September 4, 2018.
(Aug 27, 2018) ADIL releases the Lancet article signifying alcohol to be an epidemic, accounting for the #1 cause of deaths, globally, between the ages of 15-49.
(Sep 12, 2018) ADIL calls AD04, the leading drug candidate, a gene targeted therapeutic agent since 1/3 of Americans (and 50% of some European countries) have genetic predisposition for AUD. AD04 will only be utilized for this subset of population, after a proprietary genetic screening test is administered. AD04 and placebo drug stability testing results are positive, giving a 5 year maximum stability period for clinical trial purposes.
(Sep 18, 2018) ADIL successfully passes opposition period and patents AD04 in Europe (EP 2801625 B), encompassing 36 countries. It becomes evident to most investors at this point that ADIL expects to accomplish clinical trials in Europe, for FDA and EMA (European FDA equivalent) approval. The patent is expected to last until 2031. U.S. patent is set to last until 2032, before a potential Hatch-Waxman extension — a five year tack-on extension to traditional pharmaceutical patent terms, post regulatory approval.
(November 08, 2018) ADIL successfully achieves Canadian patent for AD04, through 2029-2032.
(November 19, 2018) ADIL partners with contract research organization in Europe to begin running Phase 3 trials there — FDA approved protocol is 24-weeks, 290 patients, 30 clinical sites, 5 EU countries.
(December 10, 2018) Professor Emeritus Hanno Alho, of University of Helsinki, is appointed to be the P.I. for Phase 3 Europe trials.
(December 13, 2018) ADIL makes their intentions known to expand AD04 for use in other forms of addiction as well, including Opioid Use Disorder (OUD). Quote from CEO Bill Stilley:
“We are expanding our activities around AD04 to include opioid use disorder (OUD) and believe this program has significant potential since the physiology and neuro-transmitters involved in opioid addiction are similar to alcohol and could be expected to be modulated by a serotonin-3 receptor antagonist.”
(December 24, 2018) All of ADIL’s outstanding debt obligations are retired — All convertible notes offered convert to 162,500 shares added to the common share float.
(January 14, 2019) ADIL stock price soars to $9.44, from IPO price of $5.00.
(January 24, 2019) ADIL receives $1.2 Million in capital, from exercising of warrants. A total of 368,100 shares are added to the common share float (and a total of 530,600 since IPO).
(February 15, 2019) ADIL files for U.S. patent continuation, to use AD04 for OUD purposes.
(February 21, 2019) ADIL files for share offering — 2,475,000 shares common stock + warrants (at combined price of $3.25), increasing the common share float. Warrant exercise price of $4.06 (5 yr expiry date). ADIL gross capital proceeds of $8 Million. By closing date of share offering, 2.8 million shares, in total, are added to float with gross proceeds of $9.2 Million.
(June 06, 2019) Adial Pharmaceuticals partners with Tedor Pharma, Inc. to manufacture AD04 for commercialization, post FDA approval.
(June 13, 2019) ADIL announces expected patent approval in Israel, for AD04.
(Oct 17, 2019) ADIL receives notice of approval for U.S. Patent, titled, "Serotonin Transporter Gene and Treatment of Alcoholism", for its companion diagnostic genetic test. Excerpt:
“The Patent addresses a method of identifying and treating alcohol use disorder (AUD) in patients with a specific genetic biomarker in the serotonin transporter gene. The methods include treating patients with an antagonist of the serotonin (5-HT3) receptor after detection of the biomarker in the patient. The genetic biomarker is part of the genetic panel used to identify patients for inclusion in Adial's Phase 3 trial of AD04 for the treatment of AUD in genetically targeted patients.”
(Dec 16, 2019) ADIL received additional U.S. Patent approval to build upon prior patent for companion diagnostic genetic test. This patent is for the use of AD04 along with the companion diagnostic genetic test, in patients with the genetic predisposition for AUD.
(March 17, 2020) ADIL modifies its method for recruiting and engaging with study participants to be fully online, via a telemedicine-style platform.
(April 15, 2020) ADIL receives yet another U.S. patent, covering AD04 in treatment of OUD, via companion diagnostic genetic test.
(June 15, 2020) ADIL closes share offering of 2,820,000 common stock at $1.85 per share, raising $5,217,000. Warrants also issued to purchase 2,115,000 shares (5 year expiry, exercisable $2.00 per share).
(September 30, 2020) CEO Bill Stilley says the company has $7 Million cash on hand — no immediate need for cash raise/share offering.
(November 25, 2020) ADIL enters partnership agreement with Keystone Capital, for purchasing rights of up to $15 Million worth common shares to the investment firm.
(December 10, 2020) ADIL enters agreement to acquire Purnovate — a company focused on non-opioid pain reducers and addiction treatment. Acquisition closes January 26, 2021.
(February 26, 2021) 712,500 warrants are exercised for net proceeds of $1.425 Million for ADIL.
(Mach 15, 2021) ADIL announces private placement of 700,001 shares at “above-market” price of $3.00, raising $2.1 Million.
(June 28, 2021) Adial Pharmaceuticals (ADIL) gets added to the Russell Microcap Index
(July 06, 2021) ADIL hits 100% of patient screening target (n=1254) on ONWARD Phase 3 clinical trial and 90% of patient enrollment target (n=261 of 290).
Concluding Statements
Adial Pharmaceuticals (ADIL) is in a primed position to address a major global epidemic in addiction, namely alcohol and opioids. According to the CDC, in the U.S., the annual societal cost burden of alcohol use is $249 Billion. This addiction epidemic has been aggravated by the COVID-19 pandemic. A JAMA published study has shown a 54% increase in alcohol sales during the pandemic, and a 262% increase in online sales — directly correlative to increased risk-taking behavior and mental health issues. Coincidingly, substance abuse has also skyrocketed. Currently the best available solution for AUD or OUD is through detox programs, in which patients are notorious for relapsing. ADIL has a tremendous amount of IP capital, with their licensed patent families (US 8,753,815, US 9,539,242 , and more) in over 40 countries, utilizing a well known drug (at 1/12 the lowest dose of Zofran, so adverse side-effects are minimal to none), augmented with a patented proprietary genetic screening test (which screens for the serotonin transporter gene SLC6A4).
The cost of manufacturing AD04 is very low, with estimates on tablets costing less than $0.01 per dose, and packaging/labeling costing less than $0.05 per dose.
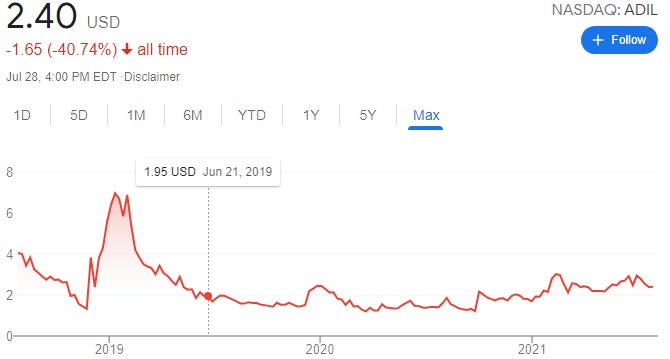
ADIL currently trades at $2.40 a share, with only 18.8 million shares outstanding, and a market capitalization of approximately $42M. As per the most recent 10-Q filing here, on March 31, 2021, ADIL has no debt, and currently sits on $5.6 Million cash on hand, with total assets eclipsing $8.6 Million. Current total liabilities are approximately $3 Million.
With the ONWARD Phase 3 trial nearing 100% in total enrollment of study participants, the clinical trial is locked and loaded to execute and soon provide top line data for the FDA and investors. With so little shares outstanding, and a high short-interest at the moment, I would not be surprised to see ADIL’s stock price increase considerably in due time.
Disclaimer: Any information presented should not be taken as investment advise, and is strictly the author’s opinion. The Content is not intended to be a substitute for professional medical advice, diagnosis, or treatment. Always seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. The sources, from which much of this information is derived, is embedded in the underlined words found in this newsletter article, giving due credit to the original source’s authors and publishers.